The mating lab addresses issues associated with sex and reproduction from an evolutionary perspective, using Tetrahymena as a model system for examining sexual and asexual reproduction. The lab takes advantage of the fact that, under appropriate conditions, Tetrahymena can propagate either vegetatively or sexually, addressing the relative impact of genetics, growth rate, and population size on reproductive advantage. Students design an experiment to identify the mating type of unknown clones of Tetrahymena by testing with clones of known mating type, based on the lack of self-mating within clones of the same mating type. The effects of various environmental conditions on mating behavior can also be examined, and the module can be expanded to include a discussion of chemotaxis and the role of surface proteins in cell-to-cell communication.
Videos
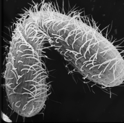
In order to conjugate, Tetrahymena cells must be starved of at least one necessary nutrient, and they must be of different mating types. There are seven mating types (I-VII) in Tetrahymena thermophila, the species shown here. Following mating, progeny cells normally are unable to mate again until they have undergone about 50 to 80 cell divisions.
This clip shows pairs of Tetrahymena during the early stages of mating (called conjugation in Tetrahymena). Early in the mating process, weakly bonded pairs are formed. These pairs can be disrupted by shaking or by adding food without damaging the cells. The reverse orientation and twisting motion is typical of cells during early mating. Since mating is not completely synchronous in any given culture of Tetrahymena cells, some boomerang-shaped pairs typical of a slightly later stage of mating are also visible. Compare the types of mating pairs in this clip to those found later in the mating process (see below).
Module Protocols
Relevant Concepts
Population dynamics; Gene regulation; Science as a Process; Reproduction and Heredity
Next Generation Science Standards Relationships
High School
HS-LS2-8 Evaluate the evidence for the role of group behavior on individual and species’ chances to survive and reproduce.
HS-LS3-2 Make and defend a claim based on evidence that inheritable genetic variations may result from: (1) new genetic combinations through meiosis, (2) viable errors occurring during replication, and/or (3) mutations caused by environmental factors.
References
●Arslanyolu M and Doerder FP. 2000. Genetic and environmental factors affecting mating type frequency in natural isolates of Tetrahymena thermophila. J.Eukaryot.Microbiol. 47 (4):412-418.
●Brown F, Tirone S, Wolfe J. 1993. Early encounters of the repetitive kind: a prelude to cell adhesion in conjugating Tetrahymena thermophila. Dev.Dyn. 196 (3):195-204.
●Doerder FP, Gates MA, Eberhardt FP, Arslanyolu M. 1995. High frequency of sex and equal frequencies of mating types in natural populations of the ciliate Tetrahymena thermophila. Proc.Natl.Acad.Sci.U.S.A. 92 (19):8715-878.
●Fujishima M, Tsuda M, Mikami Y, Shinoda K. 1993. Costimulation-induced rounding in Tetrahymena thermophila: early cell shape transformation induced by sexual cell-to-cell collisions between complementary mating types. Dev.Biol. 155 (1):198-205.
●Li S, Yin L, Cole ES, Udani RA, Karrer KM. 2006. Progeny of germ line knockouts of ASI2, a gene encoding a putative signal transduction receptor in Tetrahymena thermophila, fail to make the transition from sexual reproduction to vegetative growth. Dev.Biol. 295 (2):633-646.
●Love B and Rotheim MB. 1984. Cell surface interactions in conjugation: Tetrahymena ciliary membrane vesicles. Mol.Cell.Biol. 4 (4):681-687.
●Pagliaro L and Wolfe J. 1989. Concanavalin A inhibits mating type recognition in Tetrahymena. Exp.Cell Res. 181 (2):574-578.
●Rogers MB and Karrer KM. 1985. Adolescence in Tetrahymena thermophila. Proc.Natl.Acad.Sci.U.S.A. 82 (2):436-439.
See our glossary for the terms used in the modules.