The Phagocytosis lab uses Tetrahymena to investigate the processes of ingestion, phagocytosis, and vacuole formation in cells, and the effects of various factors on these physiological processes. In research labs, neutrophil cells can be isolated from donated blood to study how the human body combats pathogens, but that is not possible in a classroom. In this lab, students instead look at the protozoan Tetrahymena, a model organism often used in research on health and disease related topics. This safe, easily grown single-celled protist doesn’t look much like a human. Nonetheless, Tetrahymena’s basic cell processes are very much like those of human cells, and these cells can serve a model to better understand the human immune system.
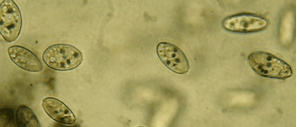
Phagocytosis video
Module Protocols
Relevant Concepts
Cellular Energetics; Chemistry of Life; Energy Transfer; Structural similarity between Single cell and Multicellular Organisms; Relationship of Structure to Function.
Next Generation Science Standards Relationships
High School:
HS-LS1-2 Develop and use a model to illustrate the hierarchical organization of interacting systems that provide specific functions within multicellular organisms.
Middle School:
MS-LS1-1 Conduct an investigation to provide evidence that living things are made of cells, either one cell or many different and types of cells.
MS-LS1-2 Develop and use a model to describe the function of a cell as a whole and ways parts of cells contribute to the function.
MS-LS1-8 Gather and synthesize information that sensory receptors respond to stimuli by sending messages to the brain for immediate behavior or storage as memories.

References
●Hoffmann EK, Rasmussen L, Zeuthen E. 1974. Cytochalasin B: aspects of phagocytosis in nutrient uptake in Tetrahymena. J.Cell.Sci. 15 (2):403-406.
●Jacobs ME, DeSouza LV, Samaranayake H, Pearlman RE, Siu KW, Klobutcher LA. 2006. The Tetrahymena thermophila phagosome proteome. 5 (12):1990-2000.
●Orias E and Rasmussen L. 1979. Dual capacity for nutrient uptake in Tetrahymena. V. Utilization of amino acids and proteins. J.Cell.Sci. 36:343-353.
●Pinheiro MD, Power ME, Butler BJ, Dayeh VR, Slawson R, Lee LE, Lynn DH, Bols NC. 2007. Use of Tetrahymena thermophila to study the role of protozoa in inactivation of viruses in water. Appl.Environ.Microbiol. 73 (2):643-649.
●Rasmussen L and Orias E. 1975. Tetrahymena: growth without phagocytosis. Science 190 (4213):464-465.
●Silberstein GB, Orias E, Pollock NA. 1975. Mutant with heat-sensitive capacity for phagocytosis in tetrahymena: isolation and genetic characterization. Genet.Res. 26 (1):11-19.
●Skriver L and Nilsson JR. 1978. The relationship between energy-dependent phagocytosis and the rate of oxygen consumption in Tetrahymena. J.Gen.Microbiol. 109 (2):359-366.
●Suhr-Jessen PB and Orias E. 1979. Mutants of TETRAHYMENA THERMOPHILA with Temperature-Sensitive Food Vacuole Formation. I. Isolation and Genetic Characterization. Genetics 92 (4):1061-1077.
●Williams NE, Tsao CC, Bowen J, Hehman GL, Williams RJ, Frankel J. 2006. The actin gene ACT1 is required for phagocytosis, motility, and cell separation of Tetrahymena thermophila. 5 (3):555-567.
See our glossary for the terms used in the modules.